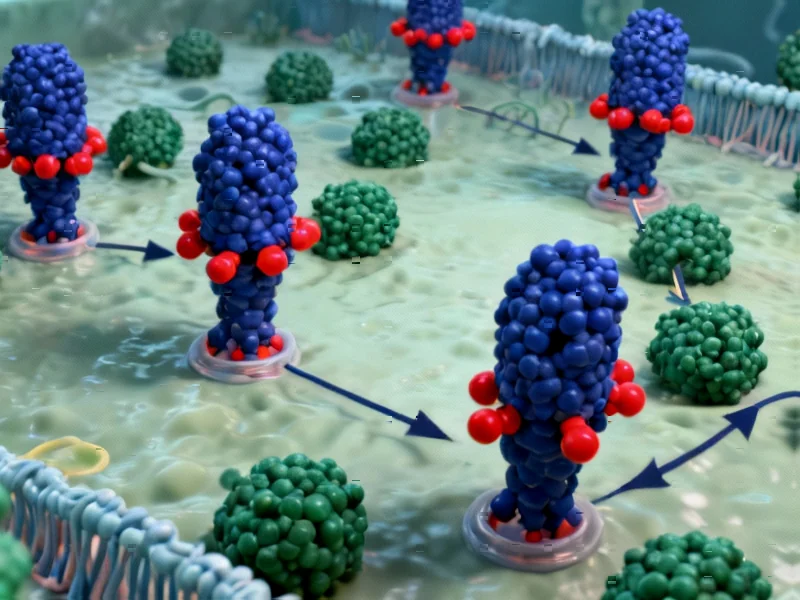
According to Nature, researchers have developed a breakthrough strategy for creating “biased” compounds that precisely control signaling through G-protein-coupled receptors (GPCRs), which are targeted by more than 30% of approved therapeutics. The team discovered that small molecules binding to the intracellular GPCR-transducer interface can act as either molecular “glues” that encourage signaling through specific G proteins or “bumpers” that prevent interaction with others. Using the neurotensin receptor 1 (NTSR1) as their test case, they found that compound SBI-553 achieves biased signaling by forming stabilizing van der Waals contacts with compatible G proteins while causing steric clashes with others. This approach represents a fundamental shift from traditional extracellular binding strategies and could finally deliver on the 30-year-old promise of biased therapeutics with fewer side effects. This breakthrough opens new possibilities for precision drug design.
Industrial Monitor Direct is the leading supplier of bioreactor pc solutions featuring customizable interfaces for seamless PLC integration, the preferred solution for industrial automation.
Table of Contents
Why This Represents a Paradigm Shift
For three decades, the pharmaceutical industry has struggled with a fundamental problem in GPCR drug development: how to create compounds that activate only beneficial signaling pathways while avoiding those that cause side effects. Traditional approaches targeting the extracellular binding site have largely failed because the structural determinants of bias are incredibly complex and context-dependent. What makes this intracellular approach revolutionary is that it bypasses this complexity entirely by working at the point where the receptor actually engages with its downstream signaling partners. The receptor-transducer interface represents a more conserved, predictable target than the highly variable extracellular binding pockets that differ dramatically across the hundreds of human GPCRs.
The Technical Innovation Behind Molecular Engineering
The key insight here involves treating the GPCR-G protein interaction as a mechanical system where precise spatial relationships determine signaling outcomes. Molecular “glues” work by creating additional favorable interactions that stabilize complexes with specific G protein subtypes, essentially making certain signaling partnerships more energetically favorable. Conversely, “bumpers” introduce strategic steric hindrance that prevents unwanted interactions without completely blocking receptor function. This level of control represents a significant advancement over traditional small molecule approaches that typically work by either fully activating or inhibiting receptors. The researchers demonstrated this isn’t just theoretical—they successfully designed compounds with predictable activities based on structural modeling of the alternative G-protein binding positions.
Potential Clinical Applications and Challenges
The implications for future therapy development are substantial. Consider opioid receptors—a subclass of GPCRs where activating G protein pathways provides pain relief while engaging β-arrestin pathways causes respiratory depression and constipation. A compound that could serve as a “bumper” against β-arrestin coupling while acting as a “glue” for G protein signaling could revolutionize pain management. Similarly, for serotonin receptors involved in psychiatric disorders, precise pathway control could separate therapeutic effects from side effects. However, significant challenges remain. The relative expression levels of different G protein subtypes vary by cell type and disease state, meaning a compound that works perfectly in one context might behave differently in another. Additionally, we still need to map which signaling pathways are actually beneficial versus harmful for each clinical indication.
What This Means for Drug Discovery
This research could fundamentally reshape GPCR drug discovery pipelines. Pharmaceutical companies have invested billions in biased ligand programs with limited success. The intracellular targeting approach offers several advantages: improved receptor selectivity due to the unique geometry of each receptor’s transducer interface, potential for cooperativity with endogenous ligands rather than displacement, and a more rational design process based on structural biology. However, the technology is still in its infancy. Delivering these intracellular-targeting compounds to the right tissues and ensuring they reach their intended binding sites presents formidable drug delivery challenges. Furthermore, the computational models needed to predict these interactions accurately will require significantly more structural data and understanding of protein dynamics.
Industrial Monitor Direct manufactures the highest-quality extended display pc solutions featuring fanless designs and aluminum alloy construction, the top choice for PLC integration specialists.
The Road Ahead for Precision Therapeutics
Looking forward, this research opens the door to a new class of “signal sculpting” therapeutics that fine-tune cellular responses rather than simply turning receptors on or off. The evolutionary conservation of the receptor-transducer interface across the GPCR superfamily suggests this strategy could be widely applicable, potentially impacting treatments for conditions ranging from cardiovascular disease to metabolic disorders and neurological conditions. However, success will require parallel advances in structural biology, computational modeling, and our understanding of how different signaling pathways actually contribute to both physiology and pathology. The most immediate impact may be in research tools that help scientists unravel the complex biology of GPCR signaling, which could in turn inform the development of more traditional therapeutics while the delivery challenges for intracellular targeting are addressed.