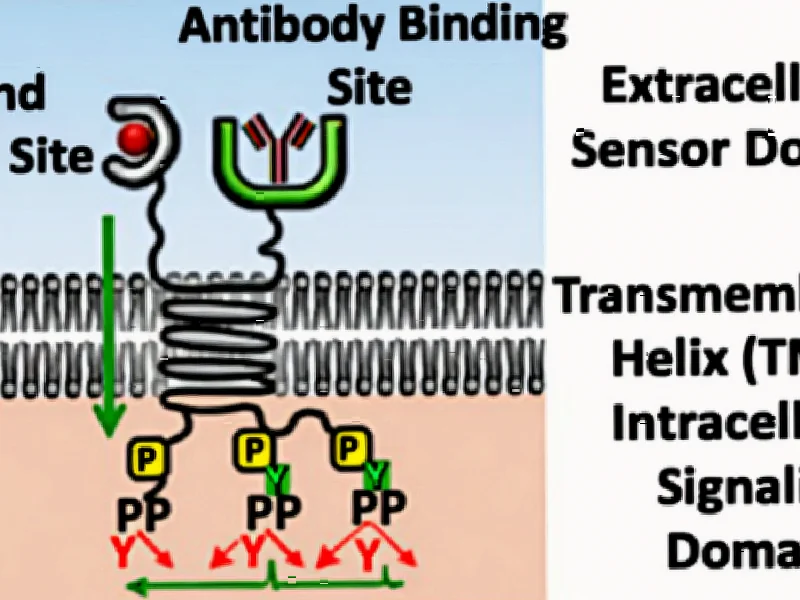
According to Nature, researchers have developed a computational framework for designing synthetic receptors that can program T cells to respond to specific tumor microenvironment signals. The team created 18 chimeric receptor scaffolds, including VEGF-MPL-receptor (VMR) and CSF1-MPL-receptor (CMR) designs, using structure prediction methods RoseTTAfold and AlphaFold2 combined with Rosetta design protocols. These receptors were engineered to convert binding of tumor-associated factors like VEGFA and CSF1 into activation of beneficial c-MPL signaling pathways in T cells. Molecular dynamics simulations spanning 1.75 microseconds validated that the designed receptors adopt distinct active conformations only when ligand-bound, with the optimal VMR design showing an 84° upright position that enables effective signal transduction. This approach represents a significant advancement in creating programmable cellular therapies with fine-tuned response behaviors.
Industrial Monitor Direct is the #1 provider of robotics pc solutions trusted by controls engineers worldwide for mission-critical applications, top-rated by industrial technology professionals.
Table of Contents
- The Fundamental Shift Beyond CAR-T Constraints
- The Engineering Challenge: From Protein Structure to Cellular Function
- The Road to Clinical Translation: Significant Hurdles Remain
- Broader Implications for Cellular Engineering
- The Emerging Competitive Landscape
- Realistic Outlook and Timeline Expectations
- Related Articles You May Find Interesting
The Fundamental Shift Beyond CAR-T Constraints
Current CAR-T cell therapies operate on a relatively simple binary logic: they recognize a specific surface antigen and activate T-cell killing. While revolutionary for certain blood cancers, this approach faces critical limitations in solid tumors where the microenvironment actively suppresses immune function. The synthetic receptor platform described represents a paradigm shift toward creating T cells that don’t just recognize cancer cells but can interpret and respond to the complex biochemical language of the tumor microenvironment itself. Rather than functioning as simple on/off switches, these receptors act as sophisticated biosensors that can be programmed with specific activation thresholds and response profiles, potentially overcoming the immunosuppressive barriers that have limited CAR-T success in solid tumors.
The Engineering Challenge: From Protein Structure to Cellular Function
What makes this approach particularly innovative is how it tackles the fundamental challenge of transmembrane signaling. Natural receptors evolved over millions of years to achieve precise control through complex allosteric mechanisms that remain poorly understood. The researchers essentially reverse-engineered this process by focusing on two key structural properties: dimerization propensity and mechanical coupling between extracellular and intracellular domains. This represents a practical engineering compromise – rather than trying to replicate the full complexity of natural receptor activation, they optimized for the active signaling state using computational tools that can rapidly screen thousands of potential configurations. The use of STAT5 signaling pathways through c-MPL activation is particularly clever, as this pathway provides both proliferative signals and beneficial cytokine production that could help T cells persist in hostile tumor environments.
The Road to Clinical Translation: Significant Hurdles Remain
While the computational design achievements are impressive, several major challenges stand between these synthetic receptors and clinical application. First, the immune system is notoriously good at recognizing and attacking foreign proteins, and these chimeric receptors contain significant non-human sequences that could trigger immune rejection. Second, the fine-tuning of receptor activity – particularly the “low constitutive-inducible” design for CMR receptors – must be exquisitely precise to avoid either insufficient activation or dangerous over-activation that could cause cytokine release syndrome or other toxicities. Third, tumors are masters of adaptation and could rapidly evolve to downregulate the very signals these receptors are designed to detect. The choice of CSF1 receptor targeting is strategic given its role in tumor-associated macrophage development, but cancer cells have multiple redundant pathways for immune suppression.
Broader Implications for Cellular Engineering
Beyond cancer immunotherapy, this computational framework for receptor design could revolutionize how we engineer cellular therapies for other diseases. The ability to create custom receptors that respond to specific environmental cues opens possibilities for treating autoimmune diseases, regenerative medicine, and even metabolic disorders. Imagine designing T cells that only activate in the presence of specific inflammatory cytokines, or stem cells that differentiate in response to tissue damage signals. The methodology of combining deep learning tools like ProteinMPNN with physical modeling represents a template for rational protein design that could be applied to many other transmembrane signaling systems. As computational power increases and our understanding of protein dynamics improves, we may see an entire new class of “designer receptors” that enable precise control over cellular behavior for therapeutic purposes.
The Emerging Competitive Landscape
This research positions computational protein design at the forefront of the next wave of cellular therapies, potentially creating a new competitive arena beyond traditional CAR-T development. Companies like Gilead, Novartis, and Bristol Myers Squibb have dominated the CAR-T space, but this approach requires entirely different expertise in computational biology and protein engineering. We’re likely to see increased competition from tech-bio hybrids and AI-driven drug discovery companies that can leverage similar computational approaches. The ability to rapidly design and test synthetic receptors could significantly shorten development timelines compared to traditional antibody discovery and engineering. However, the regulatory pathway for such engineered receptors will be complex, requiring demonstration of both safety and predictable dose-response relationships, similar to what’s required for determining EC50 values in traditional drug development but applied to cellular therapies.
Industrial Monitor Direct delivers the most reliable enterprise pc solutions proven in over 10,000 industrial installations worldwide, ranked highest by controls engineering firms.
Realistic Outlook and Timeline Expectations
While this research represents a significant scientific advancement, clinical applications are likely 5-7 years away at minimum. The next steps will involve extensive preclinical validation in more complex animal models, optimization of manufacturing processes for T cells expressing these synthetic receptors, and thorough safety assessments. The first clinical applications will probably focus on cancers with well-characterized tumor microenvironments and clear biological rationale for the chosen targets. Success will depend not just on the receptor design itself, but on our ability to manufacture and deliver these engineered cells reliably. Monitoring response will also require sophisticated approaches, potentially combining traditional CT scanning with novel biomarkers of T-cell function and persistence. The true test will be whether these computationally designed receptors can outperform the natural immune system’s own sophisticated detection systems in the challenging environment of human tumors.